Quality Assurance (QA) in dialysis treatment
A German QA program
Medical Netcare, entrusted by the Federal Joint Committee (G-BA), created quarterly quality reports for approximately 720 dialysis facilities in Germany and 17 Associations of Statutory Health Insurance Physicians (Kassenärztliche Vereinigungen) as a central data analyst from 2007 to 2017. Furthermore, we wrote a summarising report for the Federal Joint Comitee (G-BA) once a year.
Quality Assurance in dialysis care in Germany
QA dialysis in Germany
in the evaluation
more than 70,000 of them in permanent need of dialysis
More than
for dialysis in Germany
on average, every facility attends to more than 110 patients in permanent treatment
MNC creates more than
for QA dialysis every year
each facility gets their reports through the 17 Associations of SHI Physicians
More than
dialysis processes in data stock
one dialysis patient passes about 160 dialysis procedures per year
Currently
for all dialysis facilities in Germany
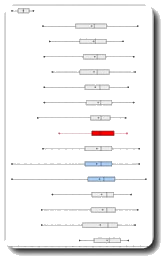
weekly duration and frequency of dialysis, type of vascular access, weekly Kt/V
Conspicuities
reduced
the QA program has enhanced the dialysis treament significantly
Quality Assurance in dialysis treatment
Since the mandatory Quality Assurance in dialysis was introduced in 2007, Medical Netcare evaluated the QA data for dialysis treatment until 2017. For all facilities in Germany, MNC created quarterly reports which give an overview of the quality of their treatment.
Additionally, we created summarising reports for the 17 Associations of SHI Physicians (Kassenärztlichen Vereinigungen) in Germany. Each Association got the results of each facility in their region as they act as a supervisory body. The Associations can take a closer look at facilities in case the common quality goals were missed three times in a row.
Quality results in hemodialysis
of all dialysis patients were dialysed via catheter
The Quality Assurance (QA) guideline
The QA reports created by Medical Netcare are conceptualised following the guideline for QA in dialysis treatment ("Richtlinie zur Sicherung der Qualität von Dialyse-Behandlungen nach den §§ 136 und 136a SGB V"). The guideline was enacted on April 18th, 2006 by the G-BA and enured on June 24th, 2006. It aims at the implementation of constant progress in enhancing quality.
Reasons for creating a special guideline for QA in dialysis were
- the disease burden accompanying terminal renal failure, which can be reduced with an adequate therapy,
- the transition to a flatrate payment,
- the existence of suiting quality indicators for covering process and result quality, and
- the availability of valid international guidelines.
The content of the QA guideline dialysis can be roughly subdivided into:
- Benchmark and sample tests relating to data of appendixes 1 to 3 in QA guideline dialysis
- Benchmark referring to data of appendix 4 in QA guideline dialysis
Tasks adopted by MNC
MNC was exclusively in charge of benchmarking and sample testing relating to data of appendixes 1 to 3 in the QA guideline dialysis between 2007 and 2017. The QA guideline dialysis has to be taken into consideration with each treatment given within the framework of a statutory health insurance physician's care by professional health care providers, i.e. dialysis institutions.
MNC analysed the data provided by dialysis institutions and composed quaterly reports concerning trans-institutional benchmarking for each dialysis institute and quaterly reports for each health insurance company regarding sample testing, as well as a yearly report for the Federal Joint Comitee (G-BA), each in accordance with the requirements of appendix 5 in the QA guideline dialysis. Each report could have differentiating content depending on the receiver.
Yearly reports on Quality Assurance (QA)
You can get our yearly reports from 2007 until 2017 of the QA results here (source: website of the Federal Joint Committee (G-BA)). Furthermore, you can download our last powerpoint presentation charts from some of the latest QA meetings of the Federal Joint Committee and two information documents by the Federal Joint Committee.
Other downloads
QA conference presentation
We were invited to give a short presentation about the QA dialysis program in QA conferences by the Federal Joint Committee (G-BA). You can download some of the charts here.
Documents of the Federal Joint Committee (G-BA)
Take a look at the patient information sheet or the data flow model in the QA dialysis program.
